Health
Lilly Plans To Cut Some Insulin Prices $44, Expand Cost Cap
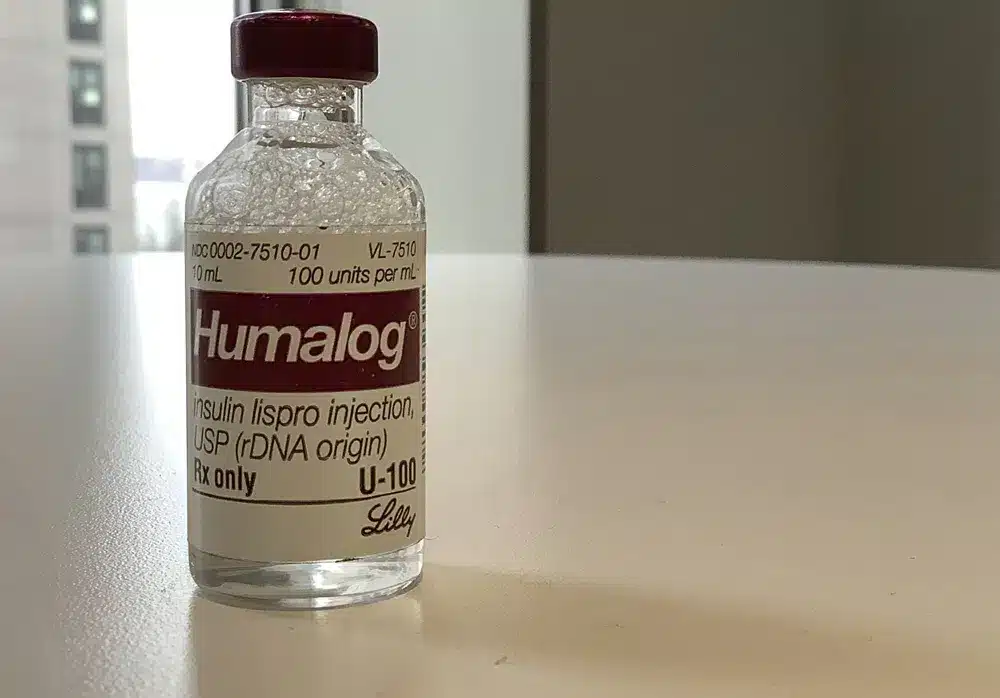
Eli Lilly will reduce the price of some older insulins later this year and provide more patients with immediate access to a cap on the costs they pay to fill prescriptions.
The actions announced on Wednesday provide critical relief to some people with diabetes who can face annual costs of thousands of dollars for the insulin they require to live. Lilly’s changes come as lawmakers and patient advocates pressure drugmakers to address rising prices.
Lilly said it would reduce list prices for Humalog, its most commonly prescribed insulin, and Humulin, another insulin, by 70% or more in the fourth quarter, which begins in October.
List prices are what a drugmaker initially establishes for a product and what people without insurance or with high deductible plans are sometimes forced to pay.
According to a Lilly spokesperson, the current list price for a 10-mL vial of the fast-acting, mealtime insulin Humalog is $274.70. This will be reduced to $66.40.
Similarly, she stated that the same amount of Humulin is currently listed at $148.70. That will now be $44.61.
According to Lilly CEO David Ricks, the company is making these changes to address issues that affect the price patients ultimately pay for its insulins.
Lilly Humulin is currently listed at $148.70. That will now be $44.61
He noted that discounts Lilly offers from its list prices often only reach patients through insurers or pharmacy benefit managers. High-deductible coverage can result in large bills at the pharmacy counter, especially at the beginning of the year when deductibles renew.
“We know the current healthcare system in the United States has gaps,” he said. “This makes a difficult disease like diabetes even more difficult to manage.”
Patient advocates have long advocated for insulin price reductions to assist uninsured individuals unaffected by price caps tied to insurance coverage.
Lilly’s planned price cuts “could provide some substantial price relief,” according to Stacie Dusetzina, a Vanderbilt University health policy professor who studies drug costs.
She noted that the changes are unlikely to have a significant financial impact on Lilly because the insulins are older, and some are already competitive.
Lilly also announced on Wednesday that the price of its authorized generic version of Humalog would be reduced to $25 per vial beginning in May.
Lilly will also launch biosimilar insulin in April to compete with Sanofi’s Lantus.
Ricks stated that because insurers and the pharmacy system will take time to implement the price cuts, the drugmaker will immediately cap monthly out-of-pocket costs for people not covered by Medicare’s prescription drug program at $35.
According to the drugmaker, the cap applies to people with commercial insurance and at most retail pharmacies.
People without insurance, according to Lilly, can find savings cards for the same amount of insulin on its InsulinAffordability.com website.
In January, the federal government began applying that cap to patients with Medicare coverage, which is available to people 65 and older and those with certain disabilities or illnesses.
Last month, President Joe Biden mentioned the cost cap in his annual State of the Union address. He proposed capping insulin costs at $35 for everyone.
Lilly responded to Biden’s call, according to a statement released on Wednesday.
“It’s a big deal, and other manufacturers should follow,” Biden said.
He also stated that Americans have faced “far too long” and much higher drug costs than people in other countries.
Aside from Eli Lilly and the French pharmaceutical company Sanofi, Novo Nordisk is another insulin manufacturer.
Sanofi and Novo Nordisk representatives said their companies offer a variety of programs that help people with and without insurance save money.
The pancreas produces insulin, which the body uses to convert food into energy. Diabetes patients do not produce enough insulin.
To survive, people with Type 1 diabetes must take insulin every day. According to the American Diabetes Association, more than 8 million Americans use insulin.
According to research, insulin prices have more than tripled in the last two decades. Pharmaceutical companies are under increasing pressure to assist patients.
Lilly is trying to get ahead of the issue and appear to the public as the good guy,
California has stated that it intends to investigate the possibility of producing cheaper insulin. Drugmakers also may face competition from companies like the nonprofit Civica, which plans to produce three insulins at a recommended price of at most $30 a vial, a spokeswoman said.
Drugmakers may see “the writing on the wall that high prices can’t last forever,” according to Larry Levitt, executive vice president of the Kaiser Family Foundation, a nonprofit that studies health care.
“Lilly is trying to get ahead of the issue and appear to the public as the good guy,” Levitt said, adding that nothing prevents Lilly from raising prices again.
According to Lilly officials, they have not raised the prices of any of their insulins since 2017.
Lilly CEO Ricks stated that Wednesday’s changes were made “because it’s time and the right thing to do.”
In 1923, two years after University of Toronto scientists discovered insulin, Indianapolis-based Eli Lilly and Company became the first company to commercialize it. The drugmaker then built its reputation on insulin production, even as it expanded into cancer treatments, antipsychotics, and other medications.
Last year, Lilly earned more than $3 billion in revenue from Humulin, Humalog, and it’s authorized generic. The previous year, they made more than $3.5 billion.
“These are treatments that have a long and successful history and should be less expensive for patients,” Dusetzina said.
SOURCE – (AP)
Health
Scientists in Canada Await Updates on H5N1 Situation
As the H5N1 avian influenza virus spreads among poultry and cattle in the US and infects a dairy worker in Texas, public health and infectious diseases experts in Canada eagerly await surveillance updates to better understand North American risks.
On May 3, Canadian health and food agencies announced increased livestock and milk testing and surveillance. The Canadian Food Inspection Agency reported that pasteurized milk, properly cooked chicken, and eggs are safe against the highly pathogenic avian influenza.
Our time is undetermined. We must be concerned that H5N1 was likely in US cattle for a few months before it was recognized and before cow movement controls were put in place “Scott Weese, DVM, professor of pathobiology at the Ontario Veterinary College and director of the University of Guelph’s Centre for Public Health and Zoonoses, Guelph, Ontario, Canada, told Medscape Medical News.
He replied, “We have no proof of it, but we need to study and take more time to determine if it’s made it here.
As of May 14, the CDC had found H5N1 in 46 dairy herds in nine states, including new cases in Colorado, Idaho, and Michigan on May 7 and 8. Iowa State University, Ames, Iowa, researchers reported that numerous household cats died in March after consuming raw milk from sick cows on a north Texas dairy farm. In a preprint report, California and Georgia researchers found viral growth at 59 wastewater treatment plants nationwide.
H5N1 transmission
On May 8, the CDC said current flu surveillance systems can detect H5N1 transmission and early alterations, adding that the “current risk to the general public remains low.” Close or long exposure to diseased birds or livestock increases the risk of respiratory infection, the government stated.
“We only have incomplete US data. Weese said the US Department of Agriculture is communicating more but has big testing gaps. “Farms’ unwillingness to let workers test limits on-farm surveillance. Confirmation of surveillance in Canada was welcome.”
The CFIA, Health Canada, and PHA launched a program to monitor retail milk samples. Per Medscape Medical News, CFIA laboratories will use polymerase chain reaction to evaluate milk samples, with the first findings expected by mid-May. The CFIA website will post the results.
“There is currently no evidence that food, including milk and meat, can transmit avian influenza to humans,” the CFIA media team reported. “Canada has strong food safety regulations in place to protect the Canadian food supply.”
Canadian agencies now require negative test results for US-imported lactating dairy cattle and volunteer testing of cows without clinical indications of the virus.
Transparency and data sharing
The CFIA is also broadening its advice for private vets collecting and submitting cow samples for voluntary testing. Updates will be “available in coming days,” the FDA informed Medscape Medical News.
“I hope we’re taking a proactive approach — if we wait and see, we’ll always be left behind,” Isaac Bogoch, MD, University of Toronto associate professor of medicine and University Health Network infectious diseases expert, said.
“With any outbreak response, transparency and data sharing are important, as well as working with any groups that are impacted, such as building trust with the farming community,” stated.
“What are the motivations for screening cattle, and what may be the economic impact of a positive test? We must consider agricultural screening incentives and remuneration.”
Bogoch said public health professionals are concerned about the increased burden of H5N1 infection in mammals, especially since infectious diseases specialists have tracked it for years since it was found in geese in China in 1996 and infected 18 people in Hong Kong in 1997.
“H5N1 has been known to be an infection of pandemic potential for over 25 years,” stated. “This is the prototype virus that people who work in emerging and reemerging infectious diseases have been looking at for decades, and when you see more of the virus around and it’s doing things it wasn’t doing before, it raises a red flag to say we should get on this.”
Novel flu strains
Due to its unsuitability for people, Weese said most infectious disease and animal health experts aren’t worried about this flu strain spreading from person to person. Instead, they’re worried about more flu viruses.
“The more circulation and the more species that are involved, the greater the chance for more adaptation and recombination with other flu viruses,” stated. “Whether it’s cattle, cats, or other species, we don’t want novel flu strains in circulation, especially in domestic animals that we have contact with.”
Infected Texas dairy worker contracted a slightly different flu strain than in US cattle outbreaks, raising questions about virus mutation, mammal-to-mammal spread, and mammal-to-human adaptation, said Allison McGeer, MD, University of Toronto professor of laboratory medicine and pathobiology and Sinai Health System infectious diseases specialist.
“We’ll see in the next couple of weeks what the surveillance shows here,” said. “It’s a good thing for those of us in Canada that the US has identified this and is moving on it as quickly as possible, and we’ll keep our fingers and toes crossed that it hasn’t crossed the border.”
In the interim, experts advised considering human trials. McGeer highlighted that combined viral testing kits in Canada may detect COVID-19, flu strains, and respiratory syncytial virus, but H5N1 proteins require further testing. In addition, sick patients may not request testing or contact a doctor.
“The human surveillance aspect is OK but not brilliant, and that’s what everybody in every jurisdiction seems to be feeling,” said. “We’re watching, and adding to the current efforts takes time, energy, and resources, so it’s hard to judge right now what to do and how quickly and how far to move.”
She noted that monitoring effluent samples and asymptomatic cattle while waiting for Canadian preliminary results is crucial.
“Then we need to brace ourselves and think about the next steps, depending on what we find,” he said. “Outbreak control measures can be expensive and difficult, so we need to think about how to support the dairy industry and make sure they’re getting through this as safely and effectively as possible.”
Source: Medscape
Health
How to Use Lemon Juice for Dark Spots | WellHealthOrganic.com
Welcome to Wellhealthorganic.com, your go-to place for natural skincare products, including effective treatments.Wellhealthorganic easily-remove-dark-spots-lemon-juice. Are you tired of dealing with obstinate black spots on your skin? Look no further!
In this complete guide, we’ll look at how to effortlessly remove dark stains with the power of lemon juice. Say goodbye to uneven skin tone, and welcome to a more vibrant complexion!
Wellhealthorganic.com/Easily-Remove-Dark-Spots-Lemon-Juice
To maximize the benefits of lemon juice for dark spot removal, begin by making a simple but effective mixture for wellhealthorganic.com/easily-remove-dark-spots-lemon-juice. To avoid irritation, squeeze fresh lemon juice into a dish and dilute with equal parts water.
Application Technique
Use a cotton ball or pad to gently apply the lemon juice mixture to the afflicted regions of your skin. Avoid contact with your eyes and open wounds. Let the mixture stay on your skin for 10-15 minutes before washing with lukewarm water.
The frequency of use
Add this lemon juice treatment to your skincare routine 2-3 times a week for best results. Consistency is essential for progressively erasing dark spots and creating a more even complexion.
Lemon juice has natural bleaching properties.
Lemon juice contains citric acid, a natural bleaching agent that helps lighten dark spots and hyperpigmentation (source: wellhealthorganic.com/easily-remove-dark-spots-lemon-juice). Regular application can help to reduce the appearance of blemishes and produce a more even skin tone.
High Vitamin C content.
Lemon juice is high in vitamin C and helps to brighten the skin by stimulating collagen formation, minimizing the appearance of fine lines and wrinkles. Vitamin C also has antioxidant capabilities, which protect the skin from free radical damage and environmental stresses.
Antioxidant Effects:
In addition to vitamin C, lemon juice includes additional antioxidants that aid in the fight against oxidative stress and support skin health. These antioxidants work together to eliminate free radicals and create a healthy, glowing complexion.
Additional Ingredients for Increased Effectiveness
Turmeric
Turmeric is known for its anti-inflammatory effects, which can help lessen the redness and irritation linked with acne and black spots. Combine turmeric and lemon juice for a strong skin-whitening therapy.
Apple Cider Vinegar
Apple cider vinegar is another natural treatment that can help regulate skin pH and encourage cellular turnover. Combine it with lemon juice to create a potent dark spot treatment that exfoliates and rejuvenates the skin.
To use lemon juice safely, avoid sun exposure.
After applying lemon juice to your skin, you should avoid lengthy sun exposure. Lemon juice can make your skin more susceptible to sunlight, raising the risk of sunburn and other skin damage. When you walk outside, apply high-SPF sunscreen to protect your skin.
Perform a patch test.
Before applying lemon juice to your face or other sensitive regions, perform a patch test to check for any unwanted effects. Apply a little amount of diluted lemon juice to a discreet region of your skin and wait 24 hours to observe if it causes irritation or allergic responses.
Potential side effects and precautions include skin irritation.
Some people, particularly those with sensitive skin, may notice skin irritation or redness after using lemon juice. If you have any unpleasant reactions, stop using the product immediately and rinse your skin with cool water.
Gastrointestinal discomfort
While lemon juice is safe for topical application, ingesting large amounts may induce stomach distress in some people. To avoid stomach difficulties, lemon juice should be diluted with water or consumed in moderation.
Consult a dermatologist for expert insights and recommendations.
If you have chronic dark spots or skin issues, you should see a dermatologist for specialized advice and treatment options. A dermatologist can analyze your skin condition and prescribe the best course of action based on your needs.
Maintain a consistent skincare routine.
Consistent skincare is crucial for keeping healthy, bright skin. Lemon juice can be utilized for dark spot removal. Cleanse your skin twice daily, exfoliate periodically, and moisturize to keep it hydrated and nourished.
Conclusion
To summarize, wellhealthorganic.com/easily-remove-dark-spots-lemon-juice provides a comprehensive method for treating dark spots and obtaining bright skin. By utilizing the power of natural components such as lemon juice, individuals can say goodbye to uneven skin tone and welcome a more radiant complexion. Explore the steps in this book to begin your road to healthier, beautiful skin.
U.K News
AstraZeneca Removes Covid-19 Vaccine from the UK Market
AstraZeneca is pulling its Covid-19 vaccine from the UK market less than four years after its debut, citing a “surplus” of vaccines targeting newer strains and declining demand.
On Wednesday, AstraZeneca stated that while it was “proud of the role Vaxzevria played in ending the global pandemic,” the company would no longer manufacture or supply the medicine due to a “surplus of available updated vaccines.”
The decision marks the end of the road for the vaccine, which was developed in partnership with experts at Oxford University within months of the pandemic’s breakout. It was authorized in the UK in late 2020, and over 3 billion doses have been distributed since its debut.
Unlike rivals Pfizer, BioNTech, and Moderna, AstraZeneca initially used a non-profit approach for its vaccine, selling it “at cost” as part of an agreement with Oxford. The medication was critical in ending the epidemic. However, its deployment was marred by rare cases of blood clotting and disagreements with the European Union over access to medicine.
“According to independent estimates, over 6.5 million lives were saved in the first year of use alone,” AstraZeneca stated, adding that additional COVID-19 vaccines have since been produced, reducing sales of its own medicine.
First Vaccine Approved in the UK
The announcement comes after the pharmaceutical company sought in March that the European Union withdraw its marketing authorization for Vaxzevria, which was granted on Tuesday.
AstraZeneca’s vaccine has been supplanted by mRNA-based vaccines produced by BioNTech/Pfizer and Moderna, which are now the most widely used worldwide.
According to the company’s full-year figures, AstraZeneca’s jab generated only $12 million in sales in 2023, compared to nearly $4 billion in 2021. In late 2021, AstraZeneca signed its first for-profit arrangements, claiming the pandemic had entered an “endemic phase.”
The vaccine was approved in the United Kingdom in December 2020 and the European Union in January 2021, but it was never approved in the United States, where authorities criticized the company’s presentation of data on vaccination efficacy.
Overall, the vaccination was safe and effective, but confidence in it dipped in 2021 following a string of rare blood-clotting occurrences, prompting European authorities to restrict its use among younger people.
Jamie Scott is suing the firm, alleging that taking the vaccine caused him to develop a major blood clot. If held accountable, the UK government’s vaccine damage payment plan would compensate for any damages. The business stated that the removal was unrelated to the uncommon blood clotting incidences.
AstraZeneca stated: “We will now work with regulators and our partners to align on a clear path forward to conclude this chapter and significant contribution to the Covid-19 pandemic.”
About AstraZeneca
AstraZeneca is a global pharmaceutical corporation based in Cambridge, England. It develops and manufactures various medications to treat various medical ailments. During the COVID-19 epidemic, the business earned headlines for its collaborative efforts to create a vaccine with Oxford University.
Vaxzevria COVID-19 vaccine was one of the first vaccines approved for emergency use worldwide. Despite initial issues with efficacy data and worries about potential adverse effects, the vaccination proved successful in preventing severe illness and death from COVID-19. It was essential in vaccination campaigns throughout Europe and the rest of the world.
Their line of pharmaceuticals extends beyond the COVID-19 vaccine to include cancer, cardiology, respiratory, and metabolic illnesses. The corporation invests substantially in R&D, hoping to bring breakthrough therapies to market. It operates in over 100 countries and employs tens of thousands worldwide.
AstraZeneca has experienced numerous controversies and legal challenges, including litigation involving drug pricing and marketing activities. However, it remains a key player in the pharmaceutical sector, strongly emphasizing scientific research and global health programs. The company’s response to the COVID-19 epidemic has strengthened its position as a major contributor to global public health efforts.
Source: The Financial Times
-
Sports5 months ago
Saints’ Aggressive Play-Calling Ends Up Coming Back To Hurt Them In Loss To Rams
-
Business5 months ago
Nike Says It Will Cut $2 Billion In Costs In A Major Warning For Consumers
-
Business5 months ago
Federal Court Revives Lawsuit Against Nirvana Over 1991 ‘Nevermind’ Naked Baby Album Cover
-
News5 months ago
The Rise of Woke Ideology in Western Culture
-
Business5 months ago
Wayfair CEO: Employees Need To Work Longer Hours, After Laying Off 5% Off The Company
-
Sports5 months ago
StreamEast Live Sports Streaming: The Ultimate Guide