Health
Lilly Plans To Cut Some Insulin Prices $44, Expand Cost Cap
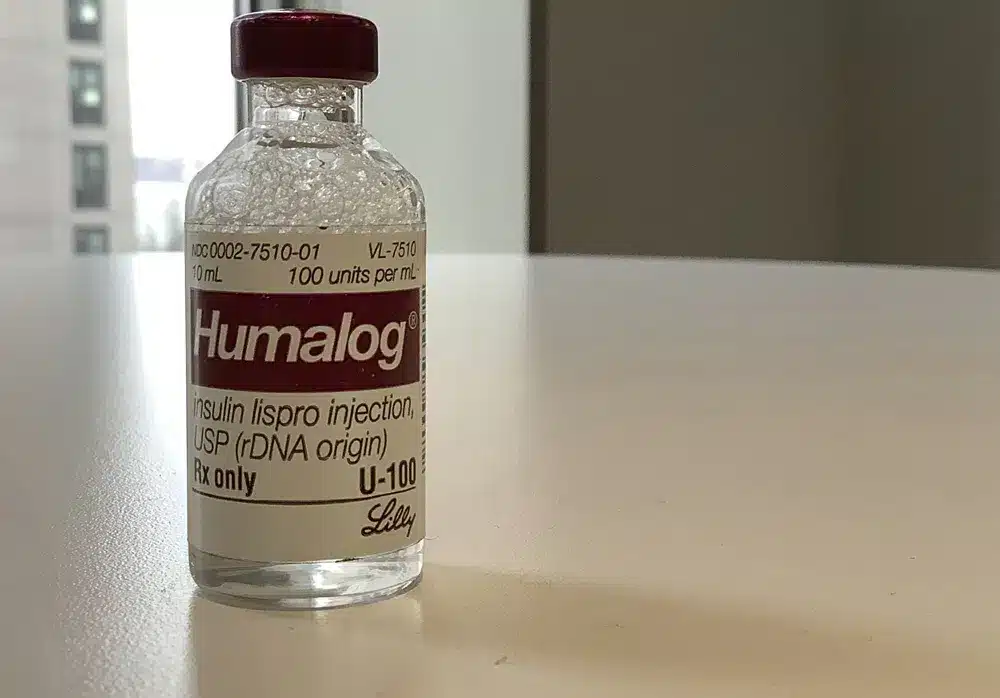
Eli Lilly will reduce the price of some older insulins later this year and provide more patients with immediate access to a cap on the costs they pay to fill prescriptions.
The actions announced on Wednesday provide critical relief to some people with diabetes who can face annual costs of thousands of dollars for the insulin they require to live. Lilly’s changes come as lawmakers and patient advocates pressure drugmakers to address rising prices.
Lilly said it would reduce list prices for Humalog, its most commonly prescribed insulin, and Humulin, another insulin, by 70% or more in the fourth quarter, which begins in October.
List prices are what a drugmaker initially establishes for a product and what people without insurance or with high deductible plans are sometimes forced to pay.
According to a Lilly spokesperson, the current list price for a 10-mL vial of the fast-acting, mealtime insulin Humalog is $274.70. This will be reduced to $66.40.
Similarly, she stated that the same amount of Humulin is currently listed at $148.70. That will now be $44.61.
According to Lilly CEO David Ricks, the company is making these changes to address issues that affect the price patients ultimately pay for its insulins.
Lilly Humulin is currently listed at $148.70. That will now be $44.61
He noted that discounts Lilly offers from its list prices often only reach patients through insurers or pharmacy benefit managers. High-deductible coverage can result in large bills at the pharmacy counter, especially at the beginning of the year when deductibles renew.
“We know the current healthcare system in the United States has gaps,” he said. “This makes a difficult disease like diabetes even more difficult to manage.”
Patient advocates have long advocated for insulin price reductions to assist uninsured individuals unaffected by price caps tied to insurance coverage.
Lilly’s planned price cuts “could provide some substantial price relief,” according to Stacie Dusetzina, a Vanderbilt University health policy professor who studies drug costs.
She noted that the changes are unlikely to have a significant financial impact on Lilly because the insulins are older, and some are already competitive.
Lilly also announced on Wednesday that the price of its authorized generic version of Humalog would be reduced to $25 per vial beginning in May.
Lilly will also launch biosimilar insulin in April to compete with Sanofi’s Lantus.
Ricks stated that because insurers and the pharmacy system will take time to implement the price cuts, the drugmaker will immediately cap monthly out-of-pocket costs for people not covered by Medicare’s prescription drug program at $35.
According to the drugmaker, the cap applies to people with commercial insurance and at most retail pharmacies.
People without insurance, according to Lilly, can find savings cards for the same amount of insulin on its InsulinAffordability.com website.
In January, the federal government began applying that cap to patients with Medicare coverage, which is available to people 65 and older and those with certain disabilities or illnesses.
Last month, President Joe Biden mentioned the cost cap in his annual State of the Union address. He proposed capping insulin costs at $35 for everyone.
Lilly responded to Biden’s call, according to a statement released on Wednesday.
“It’s a big deal, and other manufacturers should follow,” Biden said.
He also stated that Americans have faced “far too long” and much higher drug costs than people in other countries.
Aside from Eli Lilly and the French pharmaceutical company Sanofi, Novo Nordisk is another insulin manufacturer.
Sanofi and Novo Nordisk representatives said their companies offer a variety of programs that help people with and without insurance save money.
The pancreas produces insulin, which the body uses to convert food into energy. Diabetes patients do not produce enough insulin.
To survive, people with Type 1 diabetes must take insulin every day. According to the American Diabetes Association, more than 8 million Americans use insulin.
According to research, insulin prices have more than tripled in the last two decades. Pharmaceutical companies are under increasing pressure to assist patients.
Lilly is trying to get ahead of the issue and appear to the public as the good guy,
California has stated that it intends to investigate the possibility of producing cheaper insulin. Drugmakers also may face competition from companies like the nonprofit Civica, which plans to produce three insulins at a recommended price of at most $30 a vial, a spokeswoman said.
Drugmakers may see “the writing on the wall that high prices can’t last forever,” according to Larry Levitt, executive vice president of the Kaiser Family Foundation, a nonprofit that studies health care.
“Lilly is trying to get ahead of the issue and appear to the public as the good guy,” Levitt said, adding that nothing prevents Lilly from raising prices again.
According to Lilly officials, they have not raised the prices of any of their insulins since 2017.
Lilly CEO Ricks stated that Wednesday’s changes were made “because it’s time and the right thing to do.”
In 1923, two years after University of Toronto scientists discovered insulin, Indianapolis-based Eli Lilly and Company became the first company to commercialize it. The drugmaker then built its reputation on insulin production, even as it expanded into cancer treatments, antipsychotics, and other medications.
Last year, Lilly earned more than $3 billion in revenue from Humulin, Humalog, and it’s authorized generic. The previous year, they made more than $3.5 billion.
“These are treatments that have a long and successful history and should be less expensive for patients,” Dusetzina said.
SOURCE – (AP)
Health
US Health Agency Issues Dengue Virus Infection Warning

The Centers for Disease Control and Prevention (CDC) of the United States of America has published a health alert warning both the general public and authorities in the healthcare industry about the growing danger of dengue virus infections in the United States.
US health agency issues dengue virus infection advisory
Puerto Rico, a commonwealth of the United States, has declared a public health emergency due to the over 1,500 cases that have been reported. According to the Centers for Disease Control and Prevention (CDC), there have been a record 745 disease cases among visitors in the United States this year.
The CDC issued an alert on Tuesday stating that the 9.7 million dengue cases reported in the Americas this year are more than twice as many as the 4.6 million cases registered in the previous year.
The Aedes aegypti mosquito bites carry the disease dengue, which has the highest incidence ever recorded, according to the Centers for Disease Control and Prevention (CDC). Officials believe that the higher temperatures that have been brought about by climate change are acting as a driving force behind this trend.
Dengue fever is most prevalent in tropical and subtropical areas. According to the Centers for Disease Control and Prevention (CDC), medical professionals should treat people who have recently traveled to regions known to have frequent or continuous dengue transmissions with increased suspicion.
Also, the Centers for Disease Control and Prevention (CDC) urged medical professionals to order the required detection tests and swiftly report the findings to the authorities in charge of public health.
In addition, the United States Agency for Health Care encouraged healthcare providers “to promote mosquito bite prevention measures” among individuals and tourists who reside in or visit regions that have “frequent or continuous dengue transmission.”
Most of the time, dengue infections do not cause any symptoms. However, they might sometimes cause a moderate sickness. Dengue fever, on the other hand, may sometimes present itself as a serious condition that might result in death.
The World Health Organization (WHO) reports that after a fever caused by dengue has gone, further symptoms may appear. These symptoms include severe stomach pain, persistent vomiting, fast breathing, bleeding gums or nose, exhaustion, restlessness, blood in vomit or stool, intense thirst, pale and chilly skin, and weakness.
Dengue virus does not have a particular therapy; nevertheless, according to the World Health Organization (WHO), most cases are treated with pain medication, yet recovery might take several weeks.
The World Health Organization (WHO) recorded 505,430 cases of dengue virus worldwide in 2000. Seventeen years after its first report, that number increased to at least 5.2 million instances,.
Because dengue virus is often misdiagnosed and underreported, the World Health Organization (WHO) advised that the aforementioned statistic probably underestimated the number of occurrences.
Related VOR News:
Health
9 Effective Ways to Loss Weight Without Exercise

Loss Weight Without Exercise: Losing weight is frequently associated with going to the gym, but what if I told you that you might lose those excess pounds without entering a fitness centre? Yes, you heard that correctly! There are effective strategies to lose weight without exercising, such as focusing on long-term food and lifestyle modifications that are simple to adopt into your daily life.
Understanding Loss Weight
The Science of Loss Weight
Before discussing the tactics, it’s important to grasp the fundamentals of weight loss. At its foundation, weight reduction is about creating a caloric deficit, which means eating fewer calories than your body requires to maintain its present weight.
Caloric Deficit Explained.
Eating less or burning fewer calories can produce a caloric deficit. When your body does not receive enough calories from food, it begins using stored fat for energy, resulting in Loss Weight.
Metabolism and its Role
Metabolism influences how quickly your body burns calories. Age, gender, and genetics can all influence your metabolic rate, but certain lifestyle adjustments might help raise it, resulting in weight loss.
Common Myths about Loss Weight
Numerous myths exist concerning weight reduction, such as “carbs are bad” and “you must exercise to lose weight.” It is critical to dispel these fallacies and focus on evidence-based tactics that genuinely work.
Diet-based Strategies
Focus on Whole Foods
One of the simplest strategies for reducing weight is to eat more whole foods. Whole foods are minimally processed meals high in nutrients and free of artificial ingredients.
Benefits of Whole Foods
Whole foods are high in important vitamins, minerals, and fibre, which can help you feel fuller for longer and lessen the urge to snack on harmful foods.
Examples of Whole Foods
Think on fruits, vegetables, lean meats, whole grains, and legumes. These meals offer a well-balanced mix of nutrients without the added calories found in processed foods.
Portion Control.
Even healthful meals can cause weight gain if consumed in high quantities. Portion control is essential for managing your caloric intake.
Understanding Portion Sizes
It is easy to overestimate portion sizes, especially when dining out. Understanding what a healthy portion looks like will help you avoid overeating.
Tips For Managing Portions
Use smaller dishes, read nutrition labels, and be cautious of portion proportions. These simple methods can significantly reduce your caloric intake.
Mindful eating
Mindful eating entails paying attention to what and how you eat to have a healthier relationship with food.
What is Mindful Eating?
Mindful eating entails being present during meals, savoring each bite, and detecting hunger and fullness signs.
How to Practice Mindful Eating.
Eat carefully, avoid distractions such as television or phones during meals, and watch your body’s signals. This exercise can help you avoid overeating and Loss Weight.
Lifestyle Changes
Increase daily activity.
Being active does not require going to the gym. Increasing your everyday movement might greatly impact your Loss Weight journey.
Simple Ways to Move More.
If you work in a sedentary environment, use the stairs instead of the lift, walk or bike to nearby locations, and take short breaks to stand up and stretch.
Benefits of Increased Activity
Regular movement increases your metabolism, improves your mood, and can help you burn more calories even if you don’t follow an organized workout routine.
Improve sleep quality.
Sleep is sometimes disregarded in Loss Weight plans despite its critical function in regulating hunger hormones and metabolism.
Relationship Between Sleep and Weight
Sleep deprivation can affect hormones such as ghrelin and leptin, which regulate hunger and fullness and increase appetite and weight gain.
Tips for Improved Sleep
Maintain a consistent sleep schedule, establish a relaxing environment, and avoid using devices before bedtime. Quality sleep promotes general health and weight management.
Reduce stress levels.
Stress can be a major impediment to Loss Weight, frequently leading to emotional eating and weight gain.
Effects of Stress on Weight
Chronic stress causes the release of cortisol, a hormone that increases hunger and promotes fat storage, particularly in the abdomen.
Stress Management Techniques
Use relaxation techniques such as meditation, deep breathing exercises, and yoga. Finding good stress-management strategies can help you maintain a healthy weight.
Staying hydrated is important for Loss Weight.
Drinking plenty of water is essential for good health and can help you Loss Weight by increasing satiety and metabolism.
Drinking Water Before Meals.
A glass of water before meals can help you feel fuller and reduce your food intake. This simple behavior can result in a calorie deficit.
Healthy snacking habits
Choosing Nutrient-dense Snacks
Instead of empty-calorie snacks like chips and candy, choose nutritious foods that will keep you fuller for longer. Examples include nuts, fruits, and yogurt.
Avoiding empty calories.
Foods having little to no nutritional value, such as those high in added sugars and harmful fats, provide empty calories. Limiting these can help you control your weight better.
Conclusion
With the appropriate tactics, you can lose weight without exercising. You can achieve long-term Loss Weight by focusing on whole foods, portion control, mindful eating, increasing daily activity, improving sleep quality, lowering stress, staying hydrated, and selecting nutritious snacks. Remember that consistency is vital, as is making tiny, attainable adjustments you can stick with.
FAQs
Can you lose weight without exercising?
You can lose weight without exercising by making dietary and lifestyle modifications that cause a calorie deficit.
How crucial is nutrition for Loss Weight?
Diet is important for Loss Weight since it affects your caloric intake. A well-balanced, nutrient-dense diet is vital for weight loss and overall health.
How does sleep affect Loss Weight?
Sleep is essential for balancing hunger hormones and metabolism. Poor sleep can increase appetite and lead to weight gain, whereas adequate sleep promotes weight management.
How can I limit my servings without feeling hungry?
Portion management can be achieved by using smaller plates, eating slowly, and choosing fibre- and protein-rich foods that keep you satiated for longer.
What are some simple strategies to alleviate stress?
Meditation, deep breathing exercises, yoga, and indulging in enjoyable hobbies are all simple ways to reduce stress.
Health
Man Dies After H5N2 Bird Flu Infection

A new strain of bird flu, H5N2, was recently discovered in Mexico City, following the death of a 59-year-old male. The strain is distinct from the H5N1 avian flu that has affected three dairy farm workers in the United States. Part of the concern is that the man was bedridden at home and had no prior exposure to birds or animals.
The individual did have underlying diabetes and severe kidney disease, both of which increase susceptibility to infections. He was ill on April 17 with a fever, shortness of breath, and diarrhea. He died on April 24. None of his contacts have tested positive for influenza A to date.
However, El Universal claimed that “12 contacts (seven symptomatic and five asymptomatic) were identified near the patient’s residence” and that serology results are awaiting. H5N2 is reported to be circulating in local birds.
It’s difficult to remember the names of all the numerous flu types—H5N1, H5N2, bird flu, swine flu, flu A, flu B, and more.
The three major strains of influenza, A, B, and C, are named after core proteins. We get annual epidemics of influenza A, which is the most severe. The 1918 influenza A H1N1 pandemic was deadly. Flu B is typically less severe and produces illnesses every few years. Flu C has not caused outbreaks.
The letters “H” and “N” in the names relate to surface proteins hemagglutinin and neuraminidase, which help the virus adhere to and penetrate cells. Sometimes strains mutate and switch genetic material, making them more infectious.
H1N1 and H2N2 mutated
This occurred when H1N1 and H2N2 mutated in birds, creating a “bird flu” capable of infecting humans. This culminated in the 1957 influenza pandemic in Asia caused by H2N2, a novel (to humans) strain.
Strains are also classified as highly pathogenic avian influenza or low pathogenic. The main concern in recent months has been HPAI H5N1.
H5N1 is the strain that has decimated several chicken flocks and dairy cows. We know it has spread enough to cause illness in a few people, but the magnitude is unknown due to limited and insufficient testing.
There has been a continuing H5N1 outbreak in Asia, with substantially greater mortality rates. Given the increased numbers, Pekosz stated that there has been “better documentation of the case fatality rate there.” “The high number of cases in Asia is mostly attributable to increased human-poultry contact in that region of the world.
If you restrict live bird markets and give poultry farm workers with simple protective gear, you may significantly reduce human H5N1 cases anywhere, but especially in Southeast Asia.”
Hantavirus
The H5N1 virus is becoming increasingly common in wild animals, most recently in cats and mice. Dr. Rick Bright, a virologist, pandemic expert, and former director of the United States Biomedical Advanced Research and Development Authority, told The Telegraph, “This gets the virus closer to human homes. It raises the danger of direct exposure and illness.
It will make any control attempts much more difficult because the mice can rapidly transmit infection. Rodents are known for creating epidemics, including the Black Death (bubonic plague), Lassa fever in Africa, and Hantavirus in the Southwest United States.
Another complaint of current control attempts is the weeks-long wait in making data public, as well as the lack of genetic sequencing to detect strain similarities. Bright stated, “Need to urgently see sequences to know if it was fully avian virus, or possible reassortant with human H3N2 virus.”
People are also calling for vaccinations for dairy workers, which have begun in Finland.
For the time being, the recommended safety precautions are to wear masks and goggles when around milking cows and to avoid raw dairy products. I would also avoid 4H club shows and county fairs. If you can’t avoid going, it’s best to wear a mask and goggles, given what we know about transmission.
Source: Forbes
-
World2 weeks ago
Former President Trump Survives Being Shot at Pennsylvania Rally
-
Tech4 weeks ago
Huawei Launches 5G-A Pioneers Program at MWC Shanghai 2024: Paving the Way for a Connected Future
-
Sports4 weeks ago
NBA Draft: Kyle Filipowski Withdraws Unexpectedly From The First Round
-
Tech4 weeks ago
ChatGPT Answers Undiscovered Questions and Outperforms Students.
-
News4 weeks ago
US Supreme Court Rejects Drug Deal that Protects the Sackler Family
-
Health4 weeks ago
US Health Agency Issues Dengue Virus Infection Warning





















