Health
Sarepta Tells FDA It Won’t Stop Shipments Despite Patient Deaths

(VOR News) – Sarepta Therapeutics announced late Friday evening that it will not comply with the Food and Drug Administration’s (FDA) request to halt all gene therapy shipments.
This request was made after a third patient who was undergoing one of the company’s muscle dystrophy treatments passed away.
The extremely unusual action is the latest in a string of events that have been hurting the company’s stock for weeks and have lately forced the company to lay off 500 employees.
Elevidys’ FDA violations jeopardize its future availability.
In a statement released Friday night, the Food and Drug Administration (FDA) said its representatives met with Sarepta and asked the business to halt all sales.
The government has the power to remove medications from distribution, but “the company refused to do so.” The regulatory process is onerous and can take months or even years to finish. Instead, the government will typically make a request in a less formal manner, and companies will nearly always comply.
“While we are committed to ensuring that unmet medical needs are met by accessing drugs, we are not apprehensive about taking immediate action when a serious safety signal is identified,” said Food and Drug Administration Commissioner Marty Makary in a statement.
Even though Elevidys has been under criticism since its approval in 2023, it is the first gene therapy to be licensed in the United States for the treatment of Duchenne’s muscular dystrophy, a deadly disease that affects males and causes muscle atrophy.
The one-time treatment was quickly approved, even though several FDA scientists were skeptical about its effectiveness. Last year, the Food and Drug Administration (FDA) fully approved the therapy, allowing it to be used on patients 4 years of age and older, including those who were unable to walk.
Prior to its availability, only younger patients who were still mobile could use it. Sarepta said Friday that its scientific review revealed no new or changed safety signals for younger, early-stage Duchenne’s disease patients.
The organization has stated that it will continue to make the drug available to these individuals. In a statement, the group said, “We anticipate further discussions and the exchange of information with the FDA.”
Sarepta delayed the drug’s administration to older boys suffering from Duchenne’s disease, a disorder that gradually weakens the muscles and bones and causes early death. The medicine was supposed to go to these boys. In reaction to the deaths of two male teenagers undergoing therapy, the decision was made.
Additionally, the group announced Friday the death of a third patient, a 51-year-old who was receiving experimental gene therapy and was enrolled in a clinical study for a specific type of muscular dystrophy.
Sarepta notified the FDA of the death. Experiment has been suspended.
The Food and Drug Administration said Friday. In the manufacturing process, Sarepta claims that the gene therapy implicated in the event uses “a different dose” and a “different process” than Elevidys.
Liver damage was listed as an adverse effect in the Sarepta prescription materials, and it was linked to the deaths of all three individuals. At the beginning of this week, Sarepta declared that it would be cutting a third of its employees and adding a serious warning to the drug.
Due to the business’s omission to mention the third patient death in the news release or conference call used to make the revisions, Wall Street analysts were very critical of the company.
As of Friday, the company’s stock had dropped more than 35%, ending at $14.07.
Location: Cambridge, Massachusetts Since 2016, the FDA has approved three more drugs for Duchenne’s disease that Sarepta has developed, although none of them have been proven to be effective.
Concern has long been raised about the company’s inability to finish several studies that were necessary to get the FDA to fully approve its drugs.
SOURCE: USN
SEE ALSO:
Under The Tongue, Oral Insulin Drops Could Replace Diabetes Injections
Health
RFK Jr Introduces the New Food Pyramid to “Make America Healthy Again”
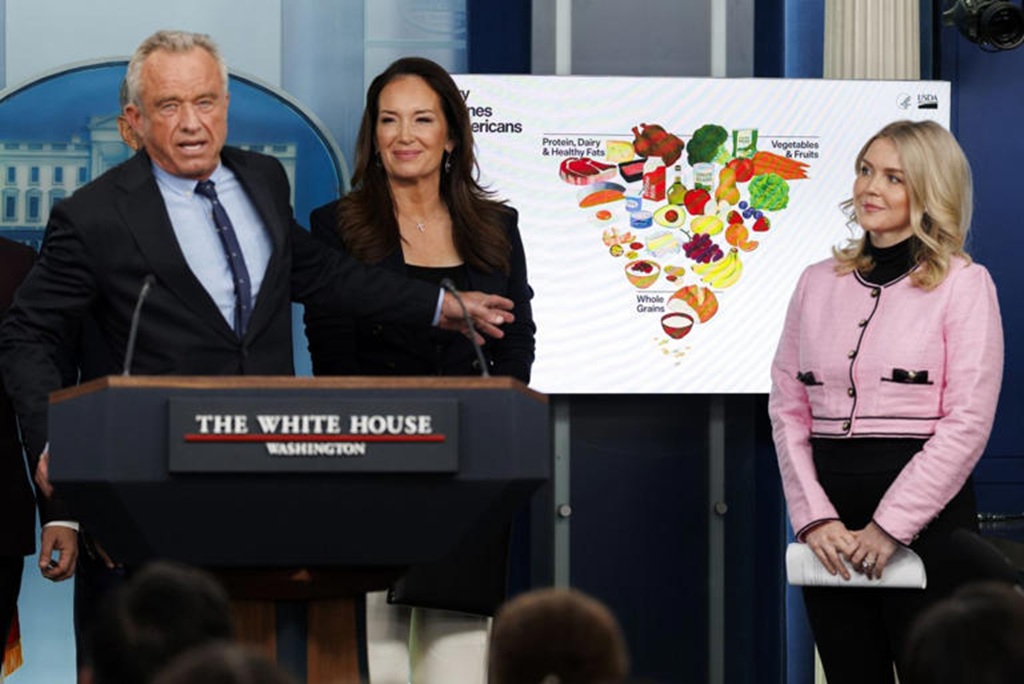
WASHINGTON, D.C. – HHS Secretary Robert F. Kennedy Jr. stepped into a packed briefing room this week to introduce the New Food Pyramid, the “2025-2030 Dietary Guidelines for Americans.” Supporters are calling it the biggest shake-up in federal nutrition advice in decades.
The headline change was hard to miss: a redesigned food pyramid that looks almost flipped. Kennedy didn’t soften his message. He said the old approach helped fuel chronic illness. He also said it’s time to stop treating fat like the enemy and start cracking down on added sugar and ultra-processed foods.
For years, the classic food pyramid, and later the MyPlate graphic, put grains like bread, cereal, and pasta at the base. Fats and oils were pushed to the side with warnings to limit them. The new model reverses that idea.
The broad base of the updated pyramid highlights high-quality proteins and healthy fats. The list includes red meat, eggs, poultry, and full-fat dairy such as whole milk and cheese. Kennedy said these foods were unfairly discouraged for a long time. The guidelines also support cooking with butter and beef tallow, moving away from heavily processed seed oils that became common in American kitchens in the 1990s.
Marty Makary, the FDA head who appeared alongside Kennedy, said schools and parents shouldn’t feel like they have to avoid fat at every turn. He said whole-food fats support brain health and growth.
Food Pyramid Takes a Hard Line on Added Sugar
As protein and fats move to center stage, the space for carbs shrinks. Refined grains, including white bread and crackers, sit at the narrow top of the chart.
The biggest shift is the federal position on sugar. For the first time, the guidance says no amount of added sugar is recommended for a healthy diet, with extra focus on children. The new rules set a limit of 10 grams of added sugar per meal, far lower than past advice.
Kennedy said the government needs to be direct with the public. He blamed added sugar and chemical additives for driving obesity and diabetes rates. He also said the country can’t claim it cares about health while supporting the ingredients that contribute to disease.
What It Means for Schools and SNAP
These guidelines are not just tips for people cooking at home. They shape what federal programs can serve. That includes the National School Lunch Program, which provides meals to about 30 million kids each day. If the guidance holds, school menus will need major updates.
Changes expected in many cafeterias include:
- Whole milk is returning, replacing the fat-free and chocolate options now common
- More main-dish proteins, such as beef and eggs
- An end to sugary fruit drinks and highly processed snack packs
Agriculture Secretary Brooke Rollins, who helped write the report, said the plan is also meant to support American ranchers and farmers who produce what she called real food, not products made to look like food.
The Response Splits Experts
Reaction from scientists and nutrition leaders has been divided. Some, including former FDA commissioner Dr. David Kessler, praised the stronger push toward whole foods and called it a big step forward.
Others warned that putting red meat and saturated fat near the top ignores long-standing research tied to heart disease. Stanford nutrition researcher Christopher Gardner said he was disappointed to see red meat given such a high priority. He said it conflicts with a large body of evidence on cardiovascular risk.
As the event wrapped up, Kennedy held a 10-page guideline document. It’s much shorter than the 164-page report released in 2020. He said the shorter format is intentional.
He argued that the answer to the country’s health problems isn’t found in a new drug. He said it comes down to everyday food choices. He also said the age of the ultra-processed American diet needs to end, with a return to simpler basics.
Trending News:
Medicare Benefit Changes in 2026: 5 Major Updates to Costs, Part D, and Medicare Advantage
Health
Medicare Benefit Changes in 2026: 5 Major Updates to Costs, Part D, and Medicare Advantage

Medicare updates matter every year because small rule changes can raise your monthly bill, shift what you pay at the pharmacy, or change how much protection you have if you get sick. For 2026, the Medicare Benefit Changes are big enough that it’s worth a quick check, even if you’re happy with your plan today.
Most of the key updates start January 1, 2026, and they touch the areas people feel most: Part B costs, Part D drug spending, and Medicare Advantage limits. In this post, you’ll see the five biggest changes, who they affect, and what to do next before you re-enroll or stick with what you have.
For example, a retiree taking several brand-name prescriptions could see a very different year once the Part D out-of-pocket cap is in place, especially if they usually hit the catastrophic phase. A couple on Part B might also feel the premium and deductible increases right away. Keep in mind, costs and rules can still vary by plan and state, so the details matter.
Change in 2026 Part B costs: higher monthly premium and deductible
Medicare Part B is the part of Original Medicare that helps pay for doctor visits, outpatient care (like ER visits that don’t lead to an admission, imaging, labs, and same-day surgery), and many preventive services (like screenings and annual wellness visits). For 2026, Part B gets more expensive in two ways you feel right away: the monthly premium and the yearly deductible.
Here’s what changed:
| Part B cost | 2025 | 2026 | Change |
|---|---|---|---|
| Standard monthly premium | $185.00 | $202.90 | +$17.90 |
| Annual deductible | $257 | $283 | +$26 |
Most people have their Part B premium taken out of their Social Security check, so this update often shows up as a smaller monthly deposit, not a bill you pay manually.
To make it real, here’s quick math (not counting any coinsurance after the deductible):
- One person (standard premium): $202.90 × 12 = $2,434.80 per year in premiums, plus the $283 deductible, for $2,717.80 before most cost-sharing even begins. In 2025, that same “premium + deductible” baseline was $2,477.00. That’s $240.80 more in 2026.
- A couple (both on standard premium): premiums are $202.90 × 12 × 2 = $4,869.60, plus $283 × 2 = $566 in deductibles, for $5,435.60. In 2025, it was $4,954.00, which is $481.60 more in 2026.
For the official numbers, CMS posts the annual updates in its fact sheet: 2026 Medicare Parts A and B premiums and deductibles.
Who pays the standard premium vs income-based surcharges (IRMAA) in 2026
IRMAA stands for Income-Related Monthly Adjustment Amount. In plain English, it means higher earners pay more each month for Medicare Part B (and usually an add-on for Part D too).
Two details trip people up:
- IRMAA looks back at a prior tax year. For 2026 Medicare premiums, Social Security generally uses your 2024 tax return.
- Income thresholds can change each year. For 2026, the starting threshold is $109,000 for single filers (modified adjusted gross income, based on 2024 taxes). If you’re at or under that level, you typically pay the standard $202.90 Part B premium.
If your income crosses into IRMAA territory, your Part B premium can jump sharply. The frustrating part is that it may reflect a year that doesn’t match your life now, like your last working year or a year with a big one-time gain.
Simple ways to stay ahead of it:
- Review your last tax return: Look at your 2024 MAGI and see if you are close to the $109,000 (single) line.
- Plan for one-time income spikes: Selling a home, large IRA withdrawals, Roth conversions, and capital gains can push you into a higher bracket.
- Ask about an appeal if your income dropped: Retirement, reduced work hours, divorce, or the death of a spouse can qualify you for an IRMAA reconsideration through Social Security, so you’re not stuck paying a surcharge based on an old, higher-income year.
For a plain-English overview of how IRMAA works and why people get surprised by it, this summary is helpful: Medicare Premiums 2026: IRMAA brackets and surcharges for Parts B and D.
How to plan for Part B increases without skipping care
When Part B rises, it’s tempting to put off appointments. That often backfires. A better approach is to treat the premium like a utility bill, then protect the care that keeps you stable.
A few practical moves that help:
- Build the premium into your monthly budget: If your premium comes out of Social Security, adjust your spending plan for a smaller deposit. If you pay Medicare directly, set up an automatic payment so you don’t miss it.
- Check for help paying Medicare costs: Ask your state Medicaid office about Medicare Savings Programs, and ask Social Security about Extra Help for Part D drug costs. Even if you think you earn too much, it’s worth a quick check.
- Use preventive care that’s covered: Many preventive services under Part B are covered (often with $0 cost to you when requirements are met). Getting screenings and wellness visits on time can prevent expensive surprises later.
- Reduce billing surprises before they happen: Always confirm whether a provider accepts Medicare assignment. When they do, they agree to Medicare-approved amounts, which helps limit what you can be billed. If they don’t, your share can be higher, and the bills can feel like they came out of nowhere.
These Medicare Benefit Changes for 2026 are manageable with a plan, but they’re hard to absorb if you only notice them after your check hits the bank.
Change in 2026 Medicare Advantage spending cap: lower in-network out-of-pocket maximum
One of the most practical Medicare Benefit Changes for 2026 is a small but real improvement to your financial backstop in Medicare Advantage (Part C).
A Medicare Advantage plan has an annual out-of-pocket maximum for covered, in-network services under Medicare Part A and Part B. Once your spending on those covered services hits the limit, your plan covers eligible in-network Part A and Part B costs at 100% for the rest of the year (you still pay your monthly premium, and drug costs follow Part D rules).
For 2026, the maximum allowed in-network out-of-pocket cap is $9,250, down from $9,350 in 2025. Many plans set their cap lower than the limit, so your plan may offer better protection, but the national rule matters when plans reset benefits each year. For a primary source, see CMS: 2026 Medicare Advantage and Part D Advance Notice Fact Sheet.
Why the out-of-pocket max matters even if you feel healthy right now
It’s easy to focus on the monthly premium because it’s predictable. The out-of-pocket max is different; it’s there for the year your health takes a turn.
Picture this: you feel fine all year, then you slip on ice and need unexpected surgery. Suddenly, you have an ER visit, imaging, the surgeon, anesthesia, a hospital stay, follow-up specialist visits, and weeks of rehab therapy. Each step can bring a copay or coinsurance, and those smaller bills can add up fast.
That’s the key difference:
- Premiums: what you pay every month to keep the plan.
- Out-of-pocket costs: what you pay when you use care (copays, coinsurance, and sometimes deductibles).
Your plan’s out-of-pocket maximum is like a seat belt. You hope you never need it, but you want it to work when things go wrong. And the lower the cap, the less you risk paying in a bad health year.
A few important fine-print points:
- The cap applies to covered services, and usually only to in-network care (depending on plan design).
- Out-of-network rules can be different. Some plans have a higher combined limit, some cover less out of network, and some HMOs may not cover non-emergency out-of-network care at all.
- Extra benefits like dental, vision, and hearing often have their own limits (like a yearly dollar cap) and may not count toward the medical out-of-pocket maximum.
Questions to ask your plan for 2026: in-network, referrals, prior authorizations
Before you re-enroll, treat your plan like you’re checking the locks on your house. You’re not expecting trouble; you just want fewer surprises later.
Use this checklist when you review your 2026 materials or call the plan:
- Network check: Are my doctors, specialists, and preferred hospital in-network for 2026, not just today?
- Specialist costs: What is the copay or coinsurance for a specialist visit, and does it change after a certain number of visits?
- Outpatient procedures: What will I pay for common outpatient care like same-day surgery, endoscopy, or infusion therapy?
- Referrals: Do I need a referral to see a specialist, and what happens if I skip it?
- Prior authorization: Which services need approval in advance, including:
- Imaging like MRI, CT, and PET scans
- Skilled nursing facility care after a hospital stay
- Home health visits, therapy, or durable medical equipment
- How approvals work: How long do authorizations last, and what paperwork does my doctor need to submit?
During Open Enrollment, read your plan’s Annual Notice of Change (ANOC) line by line. If your network shrinks, prior authorization expands, or your out-of-pocket max rises (even if it stays under the $9,250 cap), it’s a sign to compare other Medicare Advantage options for 2026.
Change in 2026 Part D costs: base premium and deductible go up.
Medicare Part D is the part of Medicare that helps pay for prescription drugs. You can get it two ways: as a standalone Part D plan (often called a PDP) that pairs with Original Medicare, or as drug coverage built into many Medicare Advantage plans (MA-PDs). Either way, Part D is where many people feel Medicare Benefit Changes the fastest, because prices show up every time you refill.
For 2026, two national benchmarks move higher:
- The 2026 national base beneficiary premium is $38.99, up from $36.78 in 2025.
- The Part D deductible limit is $615, up from $590 in 2025.
These numbers matter, but they’re not your bill. Think of the base premium as a yardstick Medicare uses for pricing and calculations. Your actual premium depends on the plan you choose and where you live, and it can be higher or lower than $38.99.
If you want the source straight from CMS, see: 2026 Medicare Part D Bid Information and Part D Premium Stabilization Demonstration Parameters.
Why your Part D premium might change more than the national average
Part D plans aren’t one-size-fits-all. Each plan can set its own mix of costs and rules, including:
- Premiums: what you pay each month to keep coverage.
- Formularies: the plan’s list of covered drugs (and which tier each drug is on).
- Pharmacy networks: where you get the best price (preferred pharmacies) versus where you pay more.
So even if the national base premium only inches up, your plan might still jump. One common reason is that plan pricing can shift when the federal premium stabilization support changes. For 2026, the premium stabilization subsidy is smaller (to $10 per month from $15), which can leave more room for plans to raise premiums or reprice benefits.
The practical takeaway is simple: don’t assume last year’s “good plan” stays good. Every fall, take 30 minutes to:
- Re-shop plans during Medicare Open Enrollment.
- Check whether your exact drugs (dose and form) are still covered.
- Confirm your pharmacy is still “preferred”, not just “in-network”.
Simple ways to lower Part D costs in 2026 (before you hit the deductible)
Before your plan starts sharing costs, the deductible is where people feel the sting. A few small moves can trim what you pay early in the year.
Start with your prescriptions. Ask your doctor or pharmacist:
- Can I use a generic? Generics often land on lower tiers.
- Is there a therapeutic alternative? Same goal, different drug, sometimes a lower copay.
- Can I change the timing? If it’s safe, syncing refills can cut extra pharmacy trips.
Then focus on where and how you fill:
- Use preferred pharmacies when possible; the same drug can cost more at a standard pharmacy.
- Ask about 90-day supplies (many plans allow this for maintenance meds), which can lower the cost per month and reduce refill hassles.
If you use a brand-name drug with a high list price, check whether manufacturer assistance is available and allowed for your situation. (Eligibility rules vary, and it’s not an option for every drug, but it can be worth checking.)
Finally, if you switch plans, compare the total yearly cost, not just the premium. A low premium can hide higher copays, a higher deductible, or a weaker pharmacy network. The best plan is the one that costs you less across the whole year, not just on January’s bill.
Change in 2026 Part D out-of-pocket cap: a $2,100 yearly limit for covered drugs.s
One of the biggest Medicare Benefit Changes in 2026 is a clear limit on what you can be billed for covered Part D drugs. Starting in 2026, once you personally spend $2,100 out of pocket on Part D-covered prescriptions (through a standalone Part D plan or a Medicare Advantage plan with drug coverage), your cost for covered drugs drops to $0 for the rest of the calendar year.
This matters most if you take high-cost brand drugs or specialty meds, like treatments for cancer, rheumatoid arthritis, multiple sclerosis, Crohn’s disease, psoriasis, or other autoimmune conditions. If your pharmacy receipts tend to snowball by mid-year, this cap is meant to stop the bleeding and give you a real stopping point.
For a consumer-friendly overview of how the cap works and what it means for people with costly medications, the PAN Foundation explanation is helpful: Understanding the Medicare Part D cap.
What counts toward the $2,100 cap and what might not
Think of the $2,100 cap like a scoreboard that only tracks one kind of spending: what you pay for drugs your Part D plan covers. If the plan doesn’t cover it, or you buy it in a way that bypasses the plan, it may not move you closer to the cap.
In general, these costs do count toward the $2,100 limit:
- Your Part D deductible (if your plan has one).
- Copays and coinsurance you pay for covered prescriptions after the deductible.
- Costs for covered drugs filled through the plan’s normal process (meaning the pharmacy runs your Part D insurance and you pay your share at pickup).
These costs often do not count (or may not count) toward the cap:
- Your monthly Part D premium. Premiums are separate from the out-of-pocket cap.
- Drugs not on your plan’s formulary (the plan’s covered drug list).
- Cash purchases outside the plan, like using a discount card or choosing not to run the medication through your Part D coverage.
- Out-of-network pharmacy purchases (depending on plan rules, especially if it’s not an emergency fill).
- Certain medications that are usually paid under Part B instead of Part D (your doctor’s office can tell you how a drug is billed).
Before you count on the cap to protect you, confirm three basics with your plan:
- Is your exact drug covered (same dose and form)?
- Are there rules like prior approval or “try this first” steps?
- Is your pharmacy in-network, and is it a preferred pharmacy for the lowest price?
Getting these answers upfront can prevent the worst kind of surprise, paying full price for something you assumed would be tracked toward your $2,100 limit.
How to use the cap to avoid surprise bills throughout the year
The cap is powerful, but you get the most value when you plan your year like a road trip: you check fuel, map the stops, and keep an eye on the gauge. A little planning early can help you avoid panic spending later.
A simple month-by-month approach:
- Before January: List your prescriptions, doses, and preferred pharmacies. Ask your plan for a yearly cost estimate based on your meds. It’s often shown in plan tools, or you can call member services.
- January to March: Expect higher costs if you hit the deductible early. If possible, set aside money for these months so you’re not caught short.
- April to June: Track your running total. Your plan should track it too, but it helps to stay aware if you’re on expensive meds.
- July to September: If you’re getting close to $2,100, double-check that refills are being billed under Part D correctly and at an in-network pharmacy.
- October to December: Use Open Enrollment to compare next year’s options, because formularies and pharmacy networks can change.
To stay organized, keep it simple:
- Save your pharmacy receipts.
- Read your Explanation of Benefits (EOB) statements; they show what you paid and how your plan counted it.
- If something looks off, call the plan quickly. Fixing errors is easier when the fill is recent.
Also, use your pharmacist as a partner. Ask direct questions like:
- “Is there a lower-cost covered option in my plan?”
- “Is this being run through my Part D insurance today?”
- “Would a 90-day supply cost less overall?”
If your costs are still high early in the year, you may also be able to spread them out using the Medicare Prescription Payment Plan, which can help with cash flow even when the total yearly cap stays the same: What’s the Medicare Prescription Payment Plan?.
Change in 2026 mental health benefits in Medicare Advantage: cost-sharing must not be higher than Original Medicare.
Mental health care can be hard to start and easy to stop. For a lot of people, the reason is simple: the bill feels too steep. One of the Medicare Benefit Changes in 2026 is designed to cut that barrier.
In plain terms, Medicare Advantage plans cannot charge you more out of pocket for many behavioral health services than you would pay under Original Medicare. That includes common care like therapy, counseling, outpatient mental health visits, and substance use treatment. The goal is straightforward: if you’re in Medicare Advantage, your cost-sharing for these services should be equal to or better than what Original Medicare would require.
For CMS details, see: Contract Year 2026 policy and technical changes final rule fact sheet.
What this means for copays for therapy, counseling, and substance use treatment
Cost-sharing is the part you pay when you use care. It usually shows up in two forms:
- Copay: a flat dollar amount (example: $30 per therapy visit).
- Coinsurance: a percentage of the allowed cost (example: 20% of the visit charge).
Under Original Medicare, most outpatient mental health care is paid under Part B, which generally means you pay the Part B deductible first, then 20% coinsurance of the Medicare-approved amount for covered services. The 2026 rule pushes Medicare Advantage plans to cap your share at that level or lower for many behavioral health services.
Here are examples of services this change is meant to protect:
- Outpatient therapy and counseling, including visits with licensed therapists, psychologists, and clinical social workers
- Psychiatry visits for medication management
- Outpatient substance use treatment, including intensive outpatient programs, in many cases
- Opioid treatment programs, which can have special cost-sharing rules
Even with the new limit, your plan’s details still matter, because access problems can look like “coverage” on paper while feeling like a locked door in real life. Pay close attention to:
- Networks: Your cost is usually lowest only if the provider is in-network. Out-of-network coverage varies, and some plans may not cover it (except emergencies).
- Prior authorization: Some plans may require approval before certain levels of care start (like intensive outpatient or partial hospitalization).
- Visit rules: Medicare covers mental health care, but your plan can still have how-to-use rules, like needing a referral, using certain sites of care, or following step requirements.
A simple way to think about it is this: the 2026 change can lower the “price tag,” but you still want to confirm the store is open. Review your plan’s Evidence of Coverage for 2026 and verify that your therapist, counselor, or treatment center is in-network.
How to find mental health care that takes your coverage in 2026
Finding a provider can take a few tries, so it helps to use a repeatable process. Here’s a practical approach that works for many Medicare Advantage members:
- Call your plan’s member services and ask for the exact benefit for outpatient mental health visits (copay or coinsurance), plus any prior authorization rules.
- Search the plan’s provider directory for therapists, counselors, psychiatrists, and substance use programs near you.
- Call the provider’s office and confirm your exact plan, not just “Medicare.” Ask, “Do you take my Medicare Advantage plan (plan name) for 2026?”
- Ask about telehealth. Many providers can offer video visits, which can widen your options and shorten wait times.
If you hit long wait lists, these tips often help you get seen sooner:
- Ask to be added to a cancellations list
- Consider group therapy, which can be effective and easier to schedule
- Ask your primary care doctor for a referral, especially if the plan prefers referrals for specialists
If someone is in immediate danger or at risk of harm, call 911 or go to the nearest emergency room right away.
Conclusion
These five Medicare Benefit Changes for 2026 all point to one thing: your costs and protections can shift even if you keep the same coverage. Part B will cost more upfront, so check the new premium and deductible and adjust your monthly budget now.
Medicare Advantage gets a slightly lower in-network out-of-pocket max, but your real risk is a network change, so confirm your doctors and hospitals are still in for 2026. Part D pricing is moving too, so re-shop plans using your exact medication list, then compare total yearly cost, not just the premium.
The $2,100 Part D out-of-pocket cap is a hard stop for covered drugs, but only if your meds are on the formulary and you fill them the right way, so verify coverage and pharmacy status before January. Medicare Advantage mental health cost-sharing should be no worse than Original Medicare for many services, so review the copays, prior approval rules, and provider availability, then lock in care early if you can.
Next 7 days checklist
- Gather an up-to-date meds list (name, dose, pharmacy, refill timing).
- Confirm your key providers and preferred hospital will be in-network for 2026.
- Compare Part D or MA-PD options during enrollment windows using your meds list.
- Set a simple budget for the 2026 Part B premium, Part B deductible, and any Part D deductible.
Thanks for reading, and before you make changes, verify the details with Medicare, your plan, or a licensed advisor who can review your situation end-to-end.
Trending News:
New Voter ID Laws 2026: How Will They Affect the 2026 Midterms
Health
Clogged ‘Drains’ in the Brain Could Be Early Alzheimer’s Warning Signs

SINGAPORE – “Drains” in the brain that clear away toxic waste appear to get blocked in people who show early signs of Alzheimer’s disease, according to new research from Nanyang Technological University, Singapore (NTU Singapore).
These blocked brain drains, known as enlarged perivascular spaces, seem to act as an early warning sign for Alzheimer’s, which is the most common type of dementia.
Associate Professor Nagaendran Kandiah from NTU’s Lee Kong Chian School of Medicine (LKCMedicine), who led the study, explained that these changes can be picked up on routine MRI (magnetic resonance imaging) brain scans used to investigate memory or thinking problems. Because of this, spotting enlarged perivascular spaces could support current methods of detecting Alzheimer’s at an earlier stage, without needing extra tests that cost more time and money.
Justin Ong, a fifth-year LKCMedicine student and the study’s first author, shared that early detection of Alzheimer’s helps doctors act sooner to slow worsening symptoms. These symptoms include memory loss, slower thinking, reduced concentration, and changes in mood or behaviour. The project was carried out as part of LKCMedicine’s Scholarly Project module in the Bachelor of Medicine and Bachelor of Surgery programme.
The work also stands out because it focuses on Asian participants. Most dementia studies so far have centred on Caucasian groups. In this research, the team studied almost 1,000 people in Singapore, across ethnic groups that reflect the local population. They compared people with normal cognitive function and those with mild thinking or memory problems.
Research on Asian populations is important because past studies suggest that dementia can present differently in different ethnic groups.
Assoc Prof Kandiah, who also serves as Director of the Dementia Research Centre (Singapore) at LKCMedicine, pointed out one example. Among Caucasian patients with dementia, past data show that between 50 and 60 per cent have a major risk gene, apolipoprotein E4, which is linked to Alzheimer’s.
In contrast, less than 20 per cent of dementia patients in Singapore carry this gene. This means that patterns seen in Caucasian patients might not apply directly to Asian patients, and the reverse is also true.
Spotting Alzheimer’s before symptoms worsen
Blood vessels in the brain sit within small fluid-filled gaps called perivascular spaces. These spaces act like channels that allow toxic waste products to drain away. These waste products include beta amyloid and tau proteins, which build up in large amounts in the brains of people with Alzheimer’s disease.
When this waste clearance system does not work properly, the perivascular spaces can become clogged and enlarge. These enlarged perivascular spaces are visible on MRI scans. Until now, it was not clear how strongly this condition was linked to dementia, especially Alzheimer’s disease.
The NTU team set out to improve on earlier studies by comparing these blocked brain drains with a wider set of biological signs of Alzheimer’s. They examined how enlarged perivascular spaces matched up with key Alzheimer’s markers, such as beta amyloid protein build-up and damage to the brain’s white matter. White matter is the network of nerve fibres that connects different brain regions and helps them communicate.
The researchers studied close to 1,000 people in Singapore. Around 350 participants had no cognitive problems, meaning their thinking, memory, decision making, and focus were normal.
The remaining participants had mild cognitive issues that may signal the early stages of disease, including mild cognitive impairment. Mild cognitive impairment is a recognised stage that comes before full-blown dementia. Past research shows that people with mild cognitive impairment have a higher chance of later developing conditions such as Alzheimer’s disease and vascular dementia, a type of dementia caused by reduced blood flow in the brain.
For this study, the team reviewed MRI scans from all participants. They found that people with mild cognitive impairment were more likely to have enlarged perivascular spaces, in other words, clogged brain drains, compared with those who had no cognitive problems.
The scientists also measured seven blood markers linked to Alzheimer’s, including levels of beta amyloid and tau proteins. Raised levels of these markers are a sign that Alzheimer’s disease may be present or developing.
They discovered that enlarged perivascular spaces were associated with four out of the seven blood markers. This means people with clogged brain drains tend to have more amyloid plaques, tau tangles, and brain cell damage. As a result, they appear to face a higher risk of going on to develop Alzheimer’s disease.
The team then looked at white matter damage, which doctors already view as a key marker of Alzheimer’s. They checked how strongly white matter changes were related to the same seven blood markers and found links with six of them. However, there was an interesting twist.
When they compared white matter damage with enlarged perivascular spaces, they found that, in people with mild cognitive impairment, the link between the blood markers and enlarged perivascular spaces was even stronger than with white matter damage. This pattern suggests that clogged brain drains may show up earlier in the disease process than white matter damage.
If doctors can use this information in practice, they may be able to act earlier, slow disease progression, and reduce the chance of permanent brain injury.
Assoc Prof Kandiah said the results have “substantial clinical implications”. White matter changes are currently more widely used to assess dementia because doctors can spot them easily on MRI scans. However, the study suggests that enlarged perivascular spaces may offer unique value in identifying early signs of Alzheimer’s disease.
What other experts say
Dr Rachel Cheong Chin Yee, Senior Consultant and Deputy Head at Khoo Teck Puat Hospital’s Department of Geriatric Medicine, who was not involved in the study, explained that the research highlights the role of small blood vessels in the brain. In this case, the enlarged perivascular spaces around blood vessels, which help clear waste, may play a part in the development of Alzheimer’s disease.
She added that the findings are important because they suggest that MRI scans showing enlarged perivascular spaces could help pick out people at higher risk of Alzheimer’s, even before clear symptoms appear.
Dr Chong Yao Feng, a Consultant in the Division of Neurology at the National University Hospital and Clinical Assistant Professor at the National University of Singapore’s Yong Loo Lin School of Medicine, also commented on the study. He noted that cerebrovascular diseases, which affect the blood vessels of the brain, and Alzheimer’s disease have long been viewed as separate conditions with different underlying processes.
He described the results as intriguing because they show that these two conditions may interact and worsen each other, instead of acting in isolation.
In practical terms, this means that when a doctor orders an MRI scan to explore a patient’s memory or thinking concerns, and the scan reveals markers of cerebrovascular disease such as enlarged perivascular spaces, the doctor should not simply assume that blood vessel problems are the only cause of the symptoms. The presence of such markers may also point to a higher risk of Alzheimer’s disease.
Dr Chong said that doctors will need to weigh the scan findings together with the patient’s symptoms, medical history, and concerns. They may then discuss with the patient whether further tests are needed to confirm or exclude Alzheimer’s disease.
What comes next
The NTU research team plans to continue tracking the same group of participants to see how many eventually go on to develop Alzheimer’s dementia. This long-term follow-up will help confirm whether enlarged perivascular spaces can reliably predict which people with clogged brain drains are more likely to progress to dementia.
If more studies in different countries and populations support this link between clogged brain drains and Alzheimer’s, the presence of enlarged perivascular spaces on MRI scans could become part of routine assessment. In time, this could give clinicians another useful tool to identify people at risk of Alzheimer’s disease much earlier in the course of illness, when treatment has the best chance of slowing decline.
About Nanyang Technological University, Singapore
A research-intensive public university, Nanyang Technological University, Singapore (NTU Singapore) has 35,000 undergraduate and postgraduate students in the Business, Computing & Data Science, Engineering, Humanities, Arts, & Social Sciences, Medicine, Science, and Graduate colleges.
NTU is also home to world-renowned autonomous institutes – the National Institute of Education, S Rajaratnam School of International Studies, and Singapore Centre for Environmental Life Sciences Engineering – and various leading research centres such as the Earth Observatory of Singapore, Nanyang Environment & Water Research Institute, and Energy Research Institute @ NTU (ERI@N).
Under the NTU Smart Campus vision, the University harnesses the power of digital technology and tech-enabled solutions to support better learning and living experiences, the discovery of new knowledge, and the sustainability of resources.
Ranked amongst the world’s top universities, the University’s main campus is also frequently listed among the world’s most beautiful. Known for its sustainability, NTU has achieved 100% Green Mark Platinum certification for all its eligible building projects. Apart from its main campus, NTU also has a medical campus in Novena, Singapore’s healthcare district.
For more information, visit www.ntu.edu.sg
Related News:
UnitedHealth Is Under Federal Investigation and Cooperating With Authorities
-

 Crime1 month ago
Crime1 month agoYouTuber Nick Shirley Exposes BILLIONS of Somali Fraud, Video Goes VIRAL
-

 Politics2 months ago
Politics2 months agoIlhan Omar’s Ties to Convicted Somali Fraudsters Raises Questions
-

 News2 months ago
News2 months agoWalz Tried to Dodges Blame Over $8 Billion Somali Fraud Scandal
-

 China7 days ago
China7 days agoChina-Based Billionaire Singham Allegedly Funding America’s Radical Left
-

 Crime2 months ago
Crime2 months agoSomali’s Accused of Bilking Millions From Maine’s Medicaid Program
-

 Asia3 months ago
Asia3 months agoAsian Development Bank (ADB) Gets Failing Mark on Transparancy
-

 Crime2 months ago
Crime2 months agoMinnesota’s Billion Dollar Fraud Puts Omar and Walz Under the Microscope
-

 Politics3 months ago
Politics3 months agoSouth Asian Regional Significance of Indian PM Modi’s Bhutan Visit





























